What is Biomaterial?
A biomaterial is a substance that has been engineered to interact with biological systems for medical purposes for therapeutic (healing, enhancing, repairing, or replacing the body's tissue functions) or diagnostic purposes. The performance and success of a medical device depends on the properties of its key biomaterial components. Therefore, the selection of the right biomaterial plays a crucial role in the development and manufacturing of medical devices.
The ideal biomaterial for use in medical devices should have the following properties: bioactive, biocompatible, non-toxic, non-corrosive, biologically inert, bioadaptable, and sterilizable.
Biomaterials are divided into four different categories, polymers, metals, ceramics, and composites, which can be used alone or in combination with each other to develop most commercially available medical devices. Biomaterials show great application promise in medical device development due to their ease of material fabrication, wide range of flexibility and biocompatibility, and their wide range of electrical, mechanical, chemical, and thermal behaviors.
Types of natural biomaterials
Chitosan
Collagen.
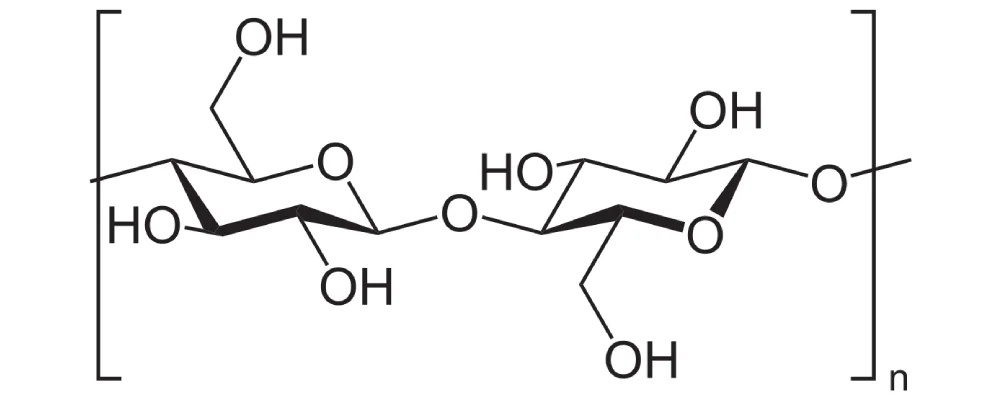
Cellulose.
Starch.
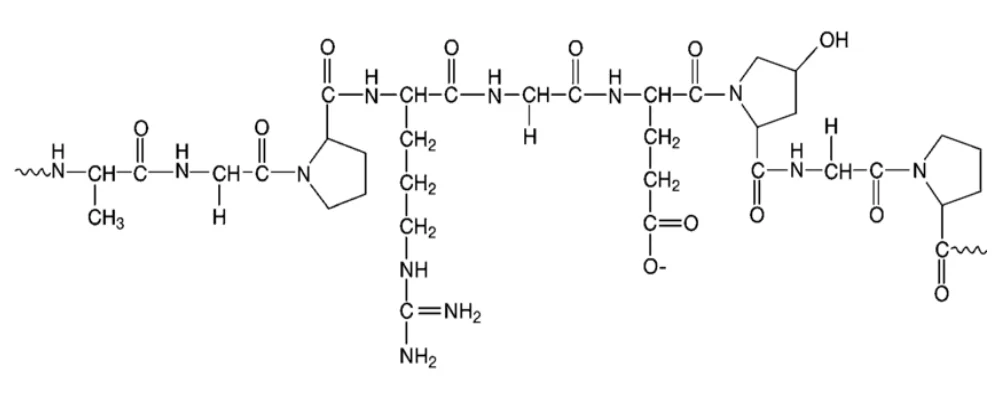
Gelatin.
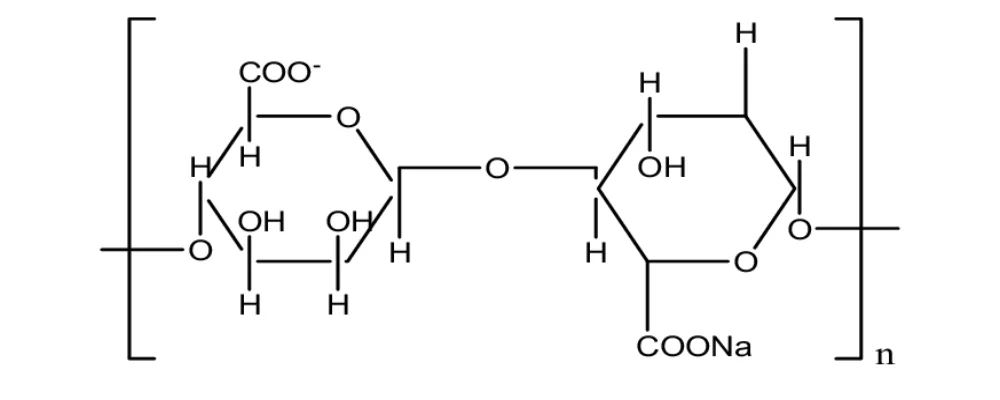
Alginic acid.
What is Chitosan?
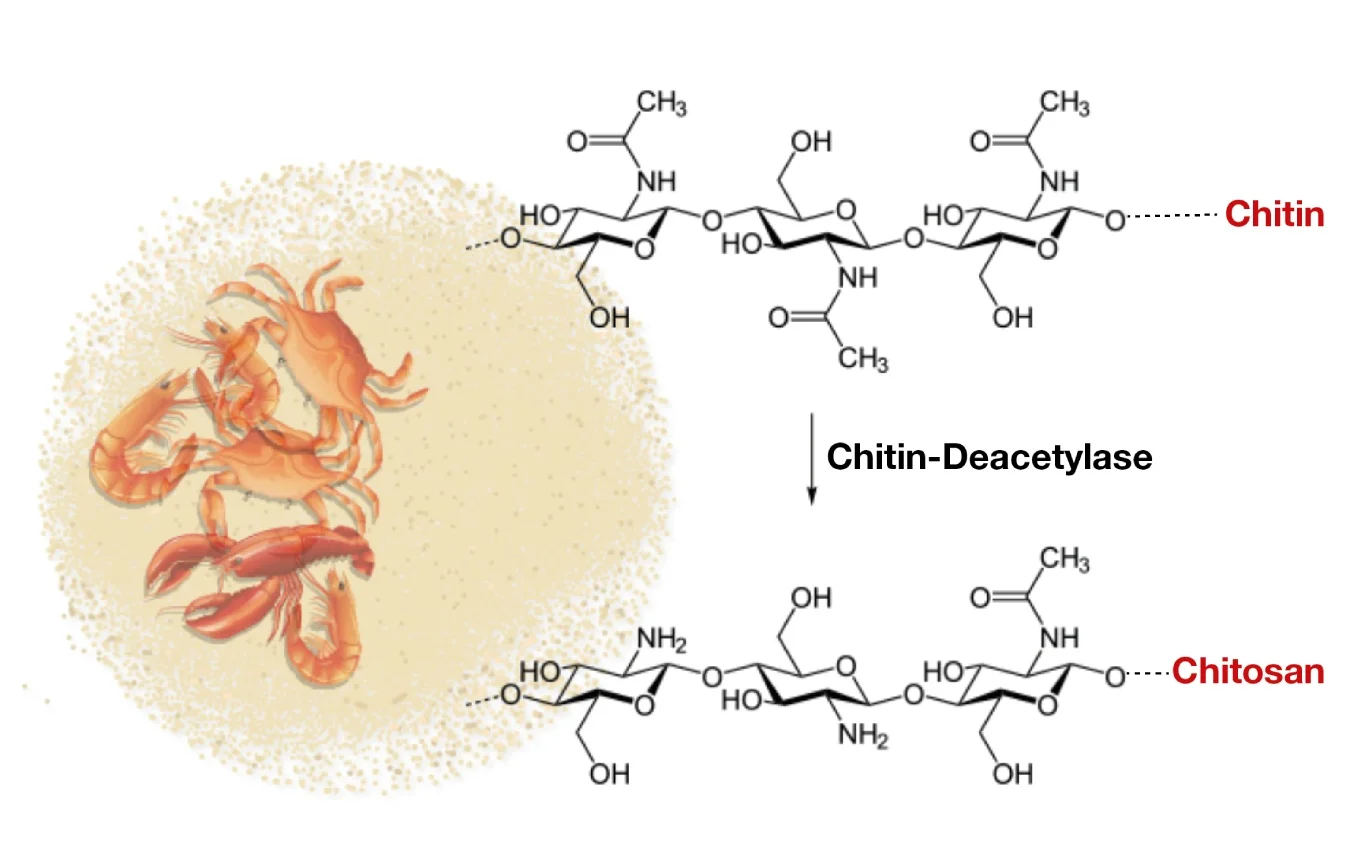
Chitosan is considered one of the promising biomaterials of the 21st century due to its versatility, excellent biodegradability, biocompatibility, antimicrobial activity, non-toxicity, and wide range of applications. Chitosan is derived from chitin, the second largest natural polymer after cellulose.
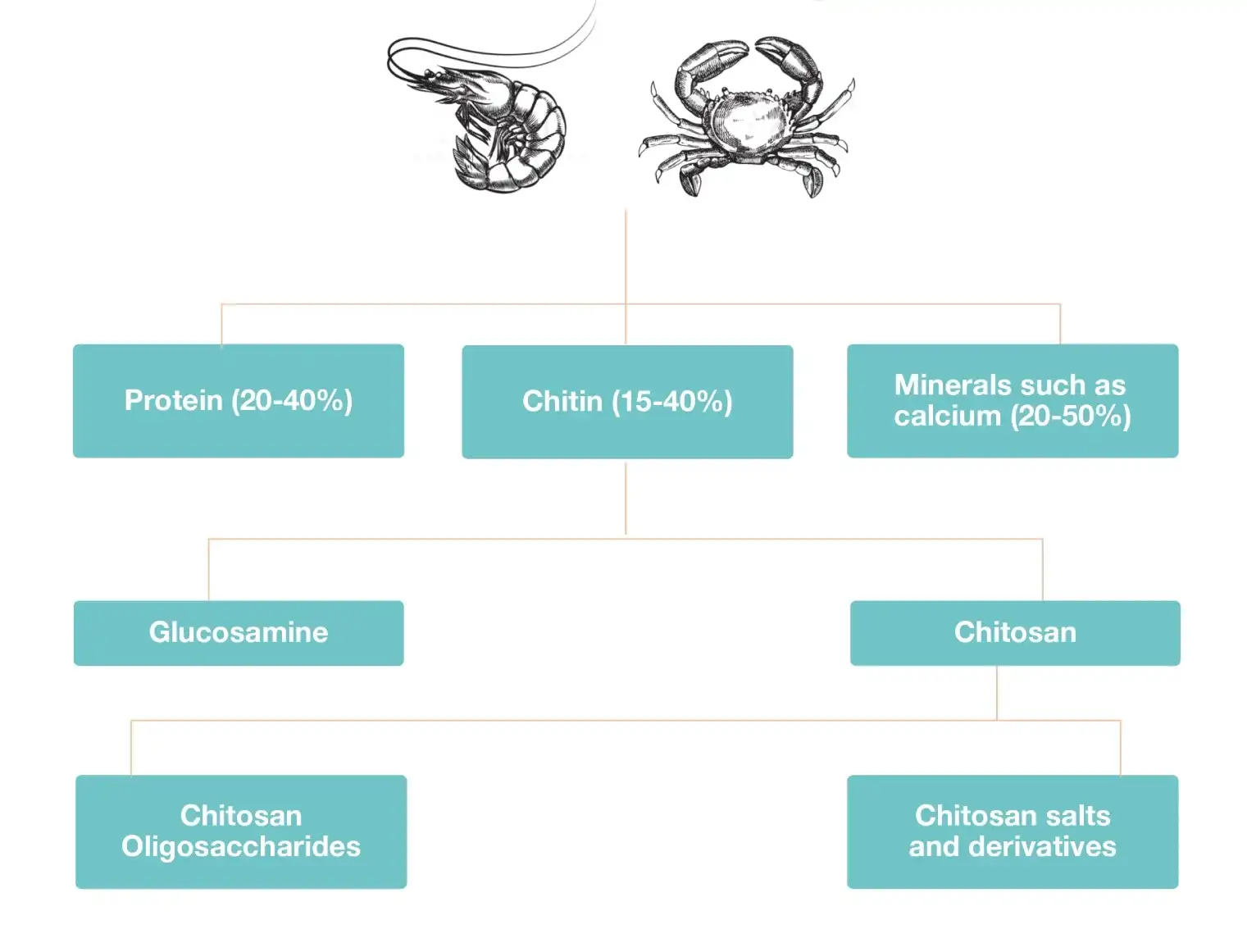
Chitin is found naturally in the exoskeleton of shellfish such as crabs and shrimp, as well as in the cell membranes of fungi, yeasts, and other microorganisms. Chitin is insoluble in dilute acids, whereas chitosan is soluble in dilute acids.
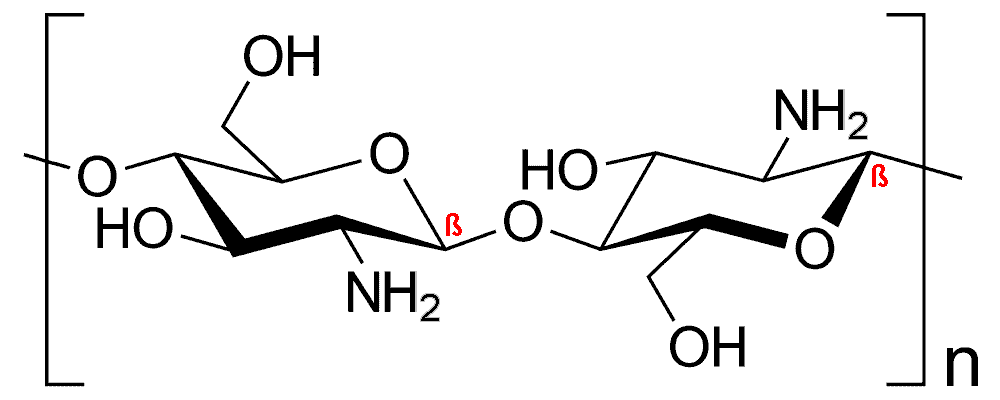
Chitosan is mainly composed of glucosamine and N-acetylglucosamine residues with a 1,4-β-bond. The presence of primary amine (-NH2) in chitosan gives it a net positive charge and is important for its biological properties.
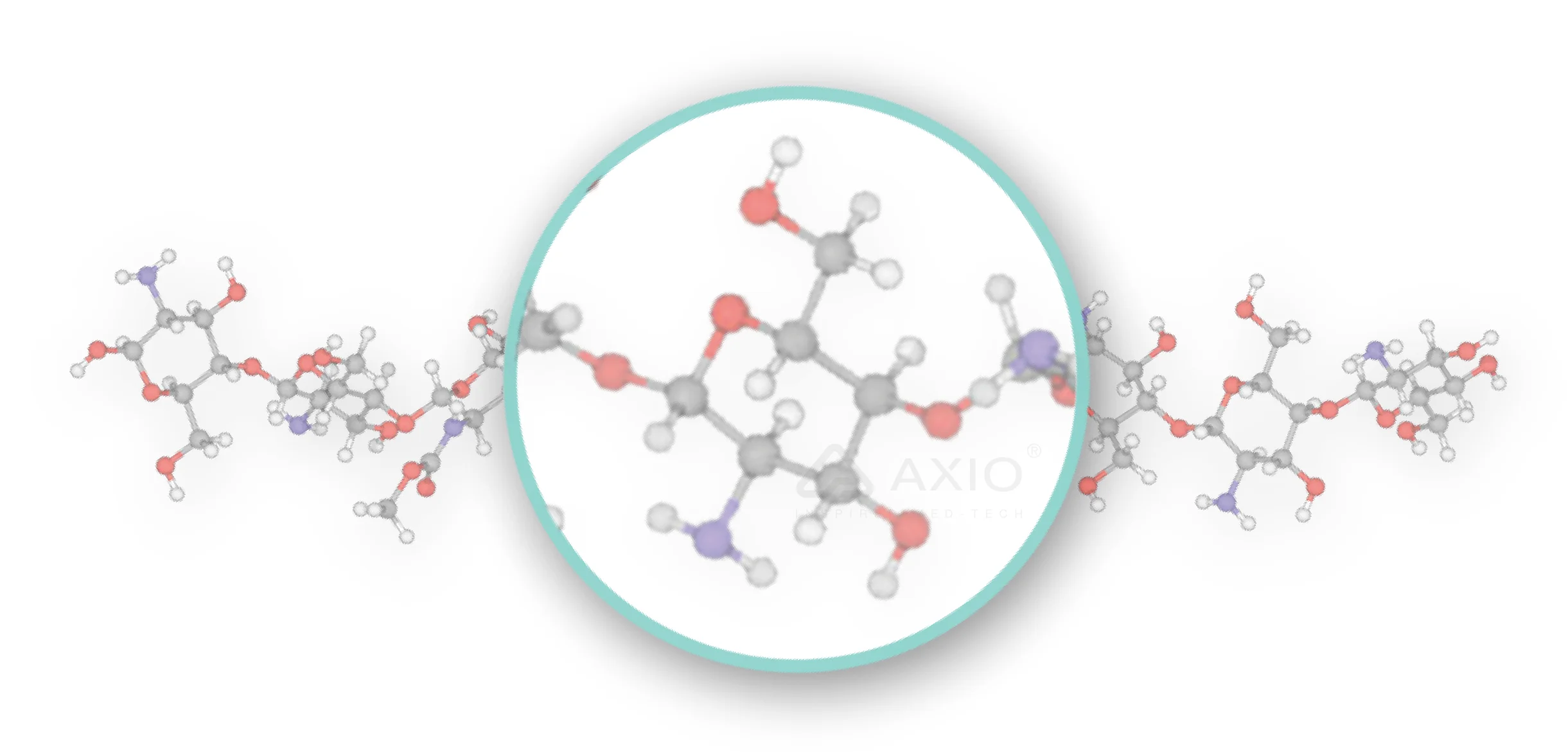
Molecular structure of chitosan
- Currently, chitosan is one of the most studied and frequently studied biomaterials
- When the degree of deacetylation (DDA) and nitrogen content in chitin exceed 50% and 7%, respectively, it is called chitosan
- Crustaceans are considered to be the main source of chitosan for commercial exploitation
What is Biomaterial?
- Chitosan is derived from chitin. It was the first polysaccharide discovered by scientists, even before the discovery of cellulose
- Chitin was first isolated from mushrooms by Henri Braconnot in 1811
- The name "Chitin" is derived from the Greek word "Chiton", which means package.
- In 1859, Professor C. Rouget first heated chitin under an alkaline medium and discovered "chitosan".
- The term "chitosan" was coined by Hoppe-Seyler in 1894
Global distribution of chitin and chitosan in nature
- Chitin is naturally distributed in the endoskeleton of cephalopods, the exoskeleton of arthropods, and the cell membranes of fungi and plants.
- Every year, 6-8 million tonnes of crustaceans are produced globally from the shells of shrimp, crab, lobster, and squid
- Countries such as India, China, Japan, Poland, Australia, the United States, and Norway have found chitin production from marine sources
Chitosan extraction
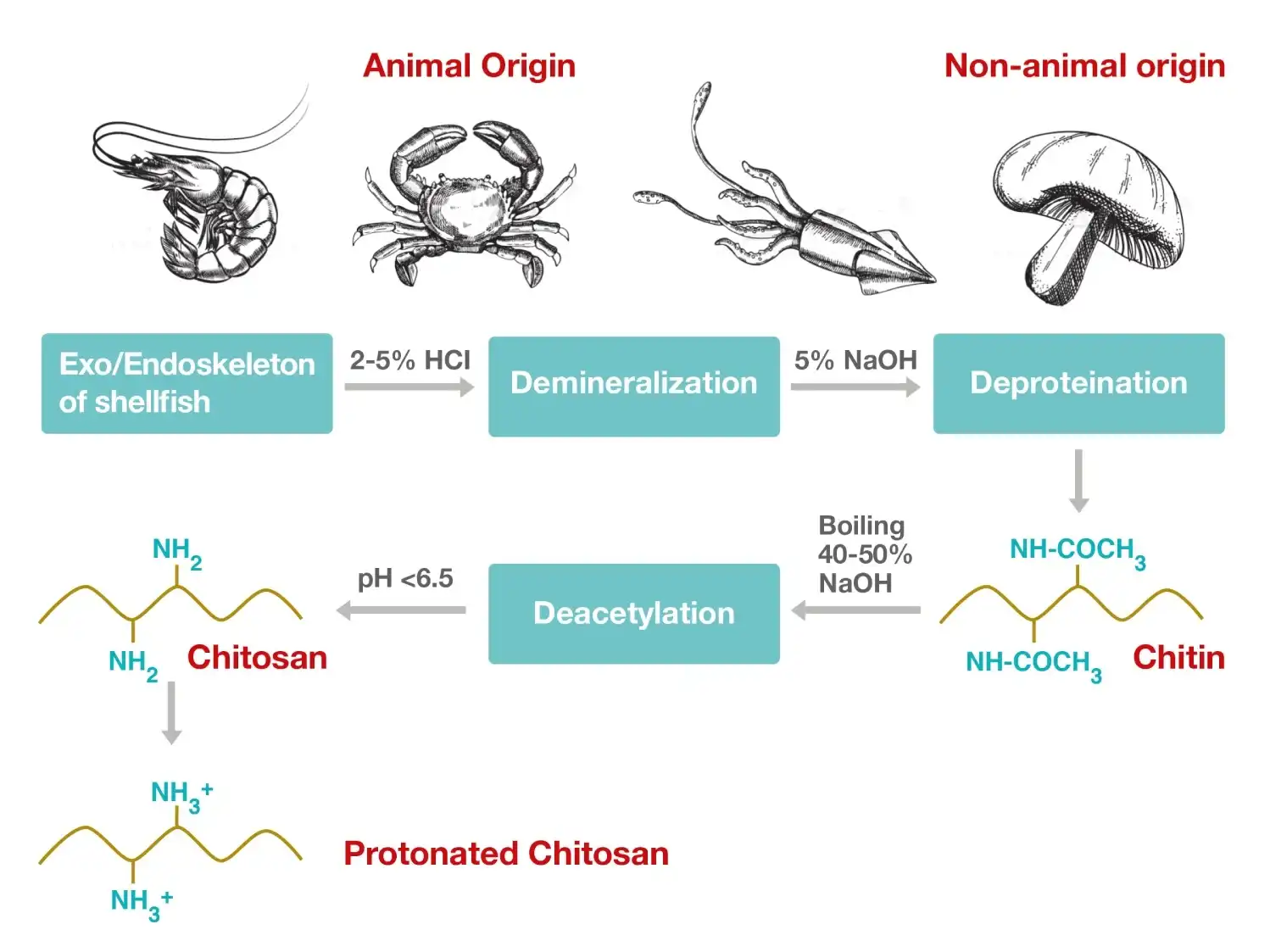
The production of chitosan starts with the selection of the right chitin extraction source. Chitin can be extracted from either animal sources (such as shellfish) or non-animal sources (such as fungi). The physicochemical properties of chitosan may vary depending on its source.
Chitin is extracted from natural sources through demineralization and deproteinization. The purified chitin is then treated with a concentrated alkali (e.g., sodium hydroxide) to obtain chitosan.
This process is known as deacetylation, and it affects the final properties of chitosan, such as the degree of positive charge (the proportion of amine groups in the polymer) and molecular weight.
Chitosan production
Types of chitosan processing
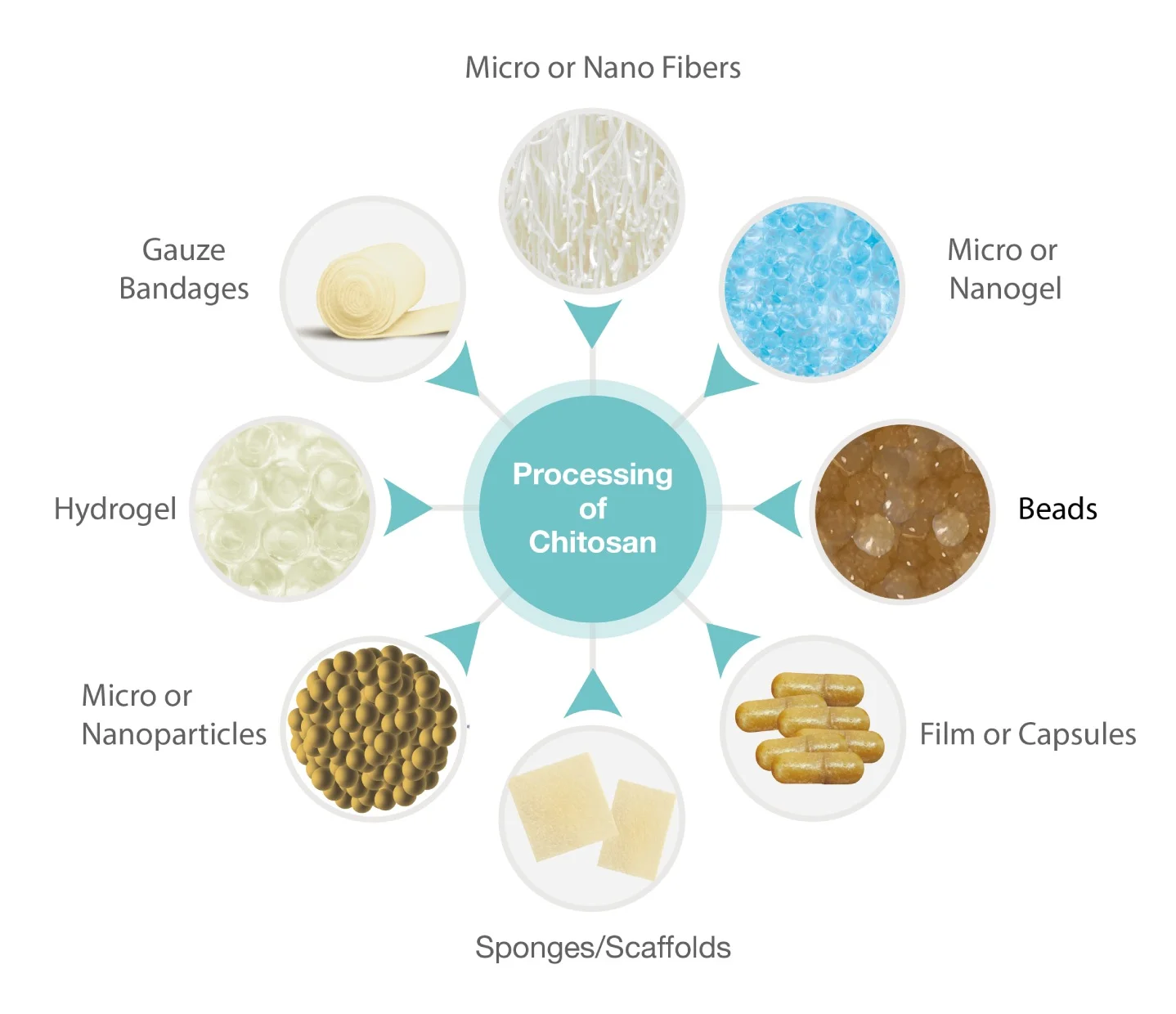
Chitosan is a widely used biomaterial. It can be processed into various forms, such as microfibers or nanofibers, micro- or nanogels, beads, films or capsules, sponges or scaffolds, micro- or nanoparticles, hydrogels, and gauze.
Properties of chitosan
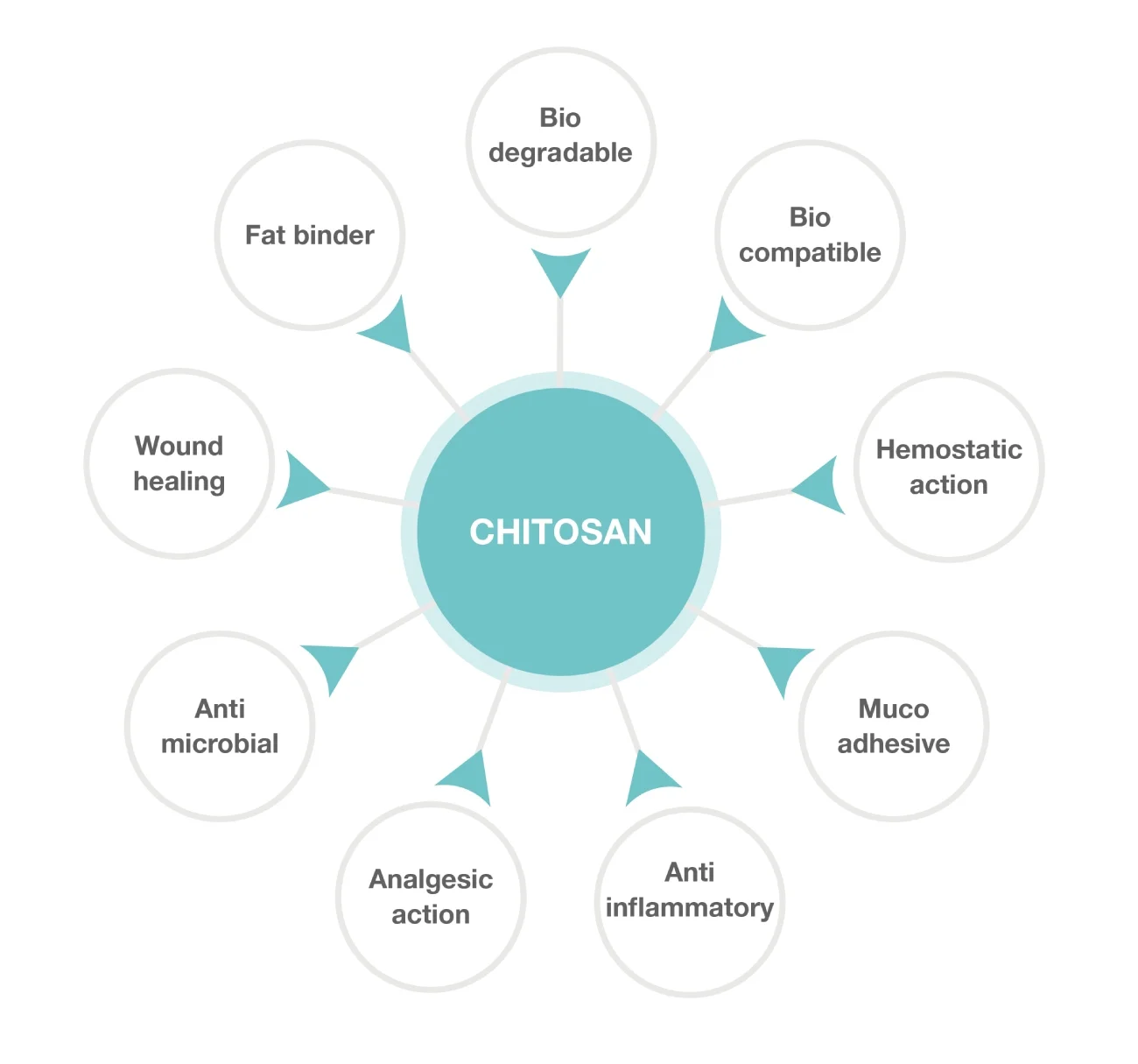
Chitosan has been extensively studied as a biomaterial used in a variety of applications. Some of its key attributes are listed in this diagram. Due to its biodegradability and biocompatibility, chitosan has been used in both external and implantable medical devices.
As a fat binder, it has been used as a weight loss agent. The adhesive and viscosity modulating properties of the mucosa allow it to be used for drug delivery. The hemostatic, antibacterial, anti-inflammatory, and analgesic properties make it an excellent wound management material.
Mechanism of important properties of chitosan
| functional | mechanism | :
|---|
| hemostatic | cationic chitosan binds to negatively charged blood cells and leads to platelet activation. |
| Antimicrobial | positively charged chitosan molecules bind to negatively charged microbial cell membranes, resulting in microbial membrane disruption and subsequent leakage of proteins and other intracellular components. |
| Pain control | chitosan relieves pain through its analgesic effect by decreasing the concentration of inflammatory mediators (bradykinin) at the site of injury. It also absorbs proton ions released from the site of inflammation to control pain. |
| Wound healing | chitosan helps in wound healing through several pathways, such as polymorphonuclear cell activation, fibroblast activation, cytokine production, giant cell migration, and stimulation of type IV collagen synthesis. |
| Prevention of scar | chitosan reduces the production of TNF-β1&2, which is responsible for scarring. The collagen produced in the presence of chitosan is tiny reticuloprotein-like fibrils rather than dense bands of mature collagen. |
Application of chitosan
Chitosan has the potential to be a game-changer in the advanced
wound care, pharmaceutical and cosmetics industries
Wound care
- The hemostatic effect of chitosan is based on the rapid absorption of plasma, resulting in the concentration of red blood cells and platelets at the site of injury, followed by platelet activation and red blood cell coagulation
- chitosan promotes re-epithelialization by stimulating the proliferation of dermal fibroblasts and inhibiting the proliferation of keratinocytes
- Chitosan acts as an antimicrobial agent by causing leakage of the bacterial cell wall and inhibiting mRNA and protein synthesis at the bacterial nucleus through charge-based interactions
- The analgesic effect of chitosan arises from the absorption of bradykinin and proton ions at the site of inflammation, followed by blockade of the arachidonic acid pathway by phospholipase A2 inhibition
- Chitosan exerts anti-inflammatory effects by producing significant inhibitory effects on inflammatory mediators such as interleukin (IL)-2, IL-4, IL-6, IL-10, and IL-13, cytokines such as TNF-α, NF-kβ expression, and AP-1 activation
- Chitosan protects the digestion of the extracellular matrix of the skin by blocking matrix metalloproteinase-2 expression
Cosmetics
- Chitosan protects the skin from UV radiation and acts as a sunscreen (SPF of about 0.89).
- Chitosan is a cationic humectant that is easily absorbed by the negatively charged skin surface and maintains the skin's moisture content. The hygroscopic capacity increases with the increase in the degree of deacetylation of chitosan
- The polycationic nature of chitosan helps to interact with polyanionic keratin, resulting in the easy formation of a thin film around the hair fiber. This film improves hair strength, softness and prevents hair damage.
The - optimal pKa value for inhibition of plaque formation is approximately 6.3, which is in the range of chitosan pKa values (5.1-6.5).
- Chitosan is able to bind to tooth enamel to form a protective layer, which reduces tooth wear
Medicines
- Chitosan is particularly used as an excipient in pharmaceutical formulations. Due to its hydrophilic nature, it can be used as a disintegrant and binder in tablet dosage forms
- The gel-forming ability of chitosan-based polymer matrices helps to control and maintain drug delivery
- Positively charged chitosan readily binds to free fatty acids and prevents lipid absorption in the intestine. Chitosan also emulsifies fats and disrupts lipid absorption in the gut
- The high molecular weight of chitosan and the presence of amino and hydroxyl groups make it suitable for use as pharmaceutical excipients such as thickeners, surfactants, emulsifiers, etc.
A key part of chitosan biomedical applications
Control bleeding and wound healing
- Natural hemostatic biomaterial
- Acts on each stage of the wound healing process
- Accelerates wound healing
- Antimicrobial effect
- Anti-inflammatory properties
- Prevent scarring
- Analgesic effect
Disease diagnosis and implants
- Bioimaging
- Biosensors
- MRI total oscillation
- Dental, orthopedic, and craniofacial implants
- Preparation of contact lenses and bone cement
Tissue Engineering and Stem Cell Technology
- Cell labeling, cell adhesion, cell encapsulation, and cell sorting
- 3D scaffolding and skin grafting
- Tissue fixation and enzyme fixation
- Blood vessels, bones, cartilage, intervertebral discs, and skin tissue engineering
Drug delivery and gene therapy
- medicine
- Mucoadhesive drug delivery
- Non-viral vector materials for gene delivery
- Controlled, continuous, and targeted
- Nanocarriers for DNA and SiRNA delivery
Wound healing
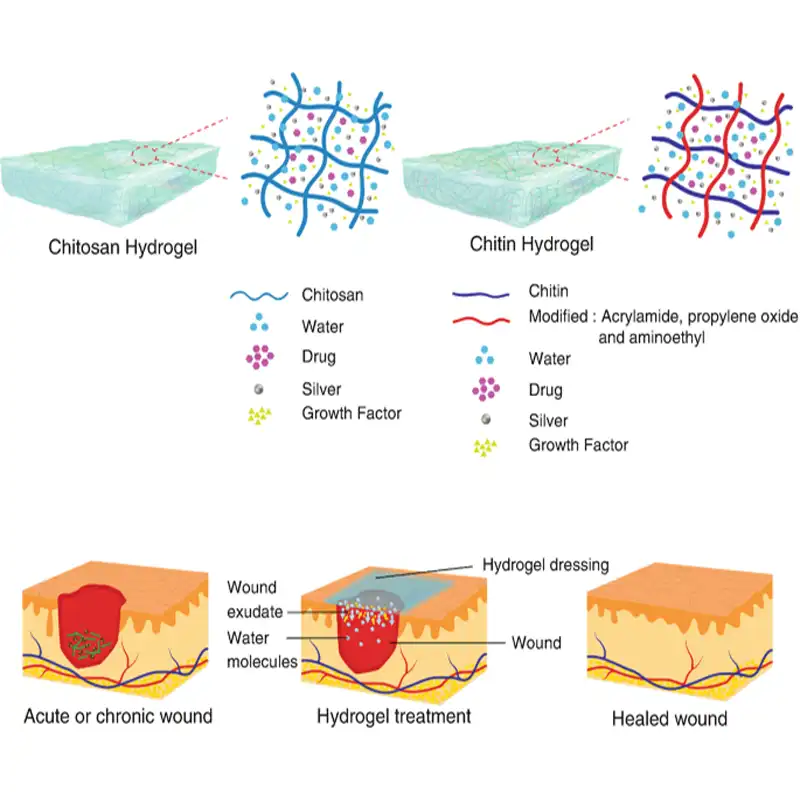
How does chitosan help with wound management?
- By protecting the wound from microorganisms
- Accelerates hemostasis
- Promotes natural wound healing
- Pain relief
- Reduces scar formation














